Root Resorption on Torqued Human Premolars Shown by Tartrate-Resistant Acid Phosphatase Histochemistry and Transmission Electron Microscopy
Abstract
Objective: To identify clastic cells on the root surfaces of torqued human premolars.
Materials and Methods: A continuous force of 600 cNmm was applied to upper first premolars in patients 13–16 years of age by using a precise biomechanical model with superelastic wires (NiTi-SE). The 28 teeth in 14 patients were divided into five groups (control [nonmoved], and moved for either 1, 2, 3, or 4 weeks) and processed for tartrate-resistant acid phosphatase (TRAP) histochemistry and transmission electron microscopy.
Results: Mononuclear TRAP-positive cells appeared at 2 weeks, wheras large multinucleated TRAP-positive cells were numerous at 3 and 4 weeks. Ultrastructural examination revealed many clastic cells in contact with resorption lacunae. In addition, some cementoblast-like cells appeared secreting new cementum over previously resorbed lacunae.
Conclusions: In general, resorption lacunae and the number of clastic cells, which increased with the duration of the applied force, were found on the cementum surface at the pressure areas. Some signs of cementum repair were also noticed, even with the maintenance of the level of the force.
INTRODUCTION
Orthodontic tooth movement requires two coupling processes that take place in the alveolar bone: resorption at the pressure side and formation at the tension side. Although only bone should be resorbed, the resorption of hard dental tissues is an undesirable side effect that frequently occurs during orthodontic treatment.1–4
Although many aspects of resorption remain unclear, it is believed that resorption involves the same pathways in all mineralized tissues through the activity of clastic cells, which are derived from mononuclear precursors that belong to the monocyte-macrophage lineage.56 Clastic cells are usually multinuclear and attach to mineralized surfaces to create resorption lacunae.7 These cells form a peripheral clear zone that is devoid of organelles, contain a well-developed actin cytoskeleton, and delimit a central area where a ruffled border is developed.7 The pH at the central area is decreased because the clastic cells pump out protons for dissolving the mineral phase.8 Then, clastic cells release some specific matrix proteases for digesting the organic components.9
Torque is a facial lingual rotation usually applied during orthodontic movement. It is measured by a relative crown and root inclination perpendicular to the line of occlusion.10 Torque is an important and potent force of the edgewise-arch mechanism and may be a potential cause of external root resorption.11–13
Although earlier studies have suggested that light and continuous forces could produce less damage on periodontal tissues,14 biomechanical systems that can maintain continuous force application have been difficult to obtain. Thus, intermittent forces have been largely used in orthodontics.15–19 Recently, superelastic nickel-titanium (Ni-Ti) wires have been developed that possess elastic memory and are capable of exerting continuous forces.20–22 However, little is known about the effects of continuous tooth movement on periodontal tissues.
We have recently showed by scanning electron microscopy that root resorption occurs in human teeth after application of continuous torque moment.23 However, the process that occurs when clastic cells arise and participate in the resorption of cementum, and eventually dentine, is not fully understood.
To test the hypothesis that clastic cells resorb the root surfaces of human teeth, a tartrate-resistant acid phosphatase (TRAP) histochemical method and transmission electron microscopy were applied to analyze the appearance and distribution of clastic cells during the application of continuous torque over several time periods.
MATERIAL AND METHODS
Subjects
Twenty-eight first upper premolars with completely developed apical thirds (stage of development 10 of Nolla24) were studied. The premolars were obtained from 14 patients (13–16 years old) and were extracted for orthodontic purposes. The study was authorized by the Ethics Committee of the University of São Paulo, Brazil.
The teeth were divided into five experimental groups: no force application (n = 4), and continuous force application for 1 week (n = 12), for 2 weeks (n = 4), for 3 weeks (n = 4), or for 4 weeks (n = 4). The samples were assigned to experimental groups to obtain an intra-individual distribution as follows:
-
Patients 1 and 2 had none of their premolars moved.
-
Patients 2–5 had their right premolar moved for 1 week and their left one moved for 2 weeks.
-
Patients 6–9 had their right premolar moved for 1 week and their left one moved for 3 weeks.
-
Patients 10–14 had their right premolar moved for 1 week and their left one moved for 4 weeks.
Biomechanics
A 600-cNmm continuous force was applied by using a precise biomechanical model. Superelastic wires (NiTi-SE Titanol, Forestadent, Pforzheim, Germany) were individually developed and calibrated by thermal treatment of the NiTi-SE portion. The biomechanical model had a stainless steel segment (0.017 X 0.022 inch), which was fixed in the molar attachment. This steel segment was connected to the superelastic segment (0.016 X 0.022 inch, NiTi-SE) that was inserted into the premolar bracket. Spring activation was achieved by twisting the superelastic segment when it was inserted into the premolar bracket slot. The pre-adjusted effective torque was 45°, which ensured the desired magnitude of moment. The molars were anchored with either a Nance appliance or transpalatal arches.23
Fixation and Decalcification
The teeth were extracted and fixed in 2% glutaraldehyde and 2.5% formaldehyde in 0.1 M cacodylate buffer at pH 7.425 for 6 hours at room temperature. The roots were separated from the crowns by using a disc, washed in the same buffer, and were decalcified in 4.13% EDTA with microwave irradiation for 50 hours.14 Then, the roots from all the experimental groups were equally divided for the two approaches (TRAP histochemistry and transmission electron microscopy) and processed as follows.
TRAP Histochemistry
Fourteen roots were transversely divided into three equal parts (heights), dehydrated in ethanol, and embedded in JB4 historesin (Polysciences Inc, Worrington, PA). Three-μm–thick sections were cut with a Microm HM360 microtome (Wolldorf, Woldorf, Germany), collected onto glass slides, and submitted to the histochemical procedure.26 Burstone's complete medium for acid phosphatase was prepared by dissolving 4 mg naphtol AS-BI phosphate substrate in 0.25 mL of N,N-dimethylformamide, followed by addition of 25 mL of 0.2 M acetate buffer at pH 5.0, 35 mg of Fast Violet LB and 60 μL of 10% magnesium chloride. The medium was filtered, and 25 mL was placed into a Coplin jar at 37°C. As a control, the substrate was omitted for half of the specimens in each group. Sodium tartrate was added at a concentration of 50 mM, and the slides were incubated for 90 minutes, washed in running water for 30 minutes, and counterstained with 10% Harris's hematoxilin. Cover slips were mounted with Euparol before examining the slides with a Nikon Optiphot-2 light microscope (Tokyo, Japan).
Transmission Electron Microscopy
Fourteen roots were transversely divided into six equal parts (heights) and subsequently trimmed longitudinally into four equal parts. They were postfixed in 1% osmium tetroxide for 2 hours, dehydrated in ethanol and acetone, and embedded in Spurr resin. Toluidine blue stained sections were examined with a light microscope, and selected regions were trimmed for cutting ultrathin sections that were collected onto copper grids, stained with lead citrate and uranyl acetate, and examined in a Jeol 1010 (Tokyo, Japan) transmission electron microscope.
RESULTS
Numerous mononuclear TRAP-positive cells were present in the periodontal ligament as early as 1 week after the application of torque. The cells were only detected at the pressure sides of the roots, at regions somewhat far from the cementum surface that appeared smooth and without signs of resorption (Figure 1). When the teeth moved for 2 weeks were examined, some multinucleated TRAP-positive cells were observed adjacent to the resorbing cementum surfaces (Figure 2).



Citation: The Angle Orthodontist 76, 6; 10.2319/071505-233



Citation: The Angle Orthodontist 76, 6; 10.2319/071505-233
After 3 weeks of torque application, multinucleated TRAP-positive clastic cells in contact with the cementum were large and numerous at the pressure side. Some regions of hyalinization of the periodontal ligament exhibited extravasation of blood cells. At these regions, clastic cells containing numerous TRAP-positive granules appeared detached from the root surface (Figure 3a). The ultrastructural examination of these cells revealed numerous vacuoles and mitochondria, but they did not exhibit a ruffled border and clear zone (Figure 3b).



Citation: The Angle Orthodontist 76, 6; 10.2319/071505-233
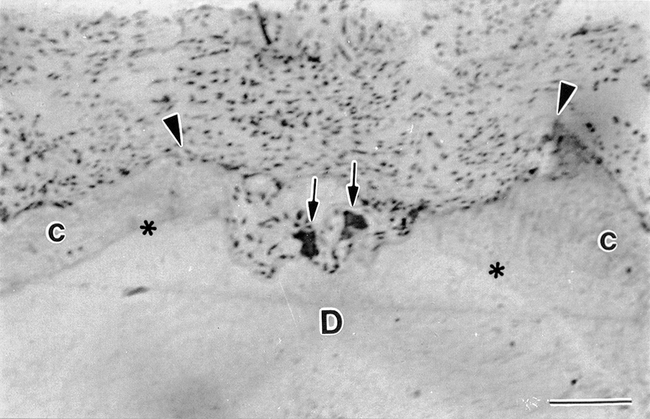
After 4 weeks of tooth movement, most clastic cells were attached to deep lacunae, many of them reaching the underlying root dentine (Figure 4). The cells exhibited numerous TRAP-positive granules inside their cytoplasm (Figures 5a and 6a) and were detected in contact with resorbing root surfaces containing many mitochondria and vacuoles and displaying a well-developed ruffled border. However, they rarely presented a clear zone (Figures 5b and 6b).


Citation: The Angle Orthodontist 76, 6; 10.2319/071505-233



Citation: The Angle Orthodontist 76, 6; 10.2319/071505-233



Citation: The Angle Orthodontist 76, 6; 10.2319/071505-233
In some regions on the pressure sides, signs of repair were detected concomitant with tissue damage and resorption of the root surface. Some deeply resorbed lacunae contained one or two large multinuclear TRAP-positive clastic cells. However, they were not in contact with the root surface and some cementoblast-like cells were often interposed between the clastic cells and the root surface. In addition, the cells and matrix of the periodontal ligament contained in these concavities showed no signs of alteration (Figures 7a and 7b). Ultrastructural analysis revealed that these cells interposed between the clastic cells and the previously resorbed lacunae of the root surface displayed many synthesis organelles. In several lacunae, cementoblast-like cells were observed over a newly secreted organic matrix (Figure 7c).



Citation: The Angle Orthodontist 76, 6; 10.2319/071505-233
DISCUSSION
The present study revealed the presence of clastic cells after the application of a continuous torque on human teeth. The cells generated numerous areas of resorption at the pressure sides of the root, and the degree of severity was related to the duration of the force application. In addition, some signs of repair were noticed on previously resorbed areas.
Clastic cells are microscopically recognized as multinucleated giant cells located in resorbing concavities on mineralized tissue surfaces. However, mononuclear clastic cells have been found, especially when dentine is resorbed, during deciduous tooth shedding.27 As in these cases, the identification of clastic cells by routine histological methods may be difficult. The histochemical detection of the TRAP enzyme has been shown to be a suitable approach for the microscopic identification of clastic cells and their precursors. The TRAP staining is considered specific for clastic cells. However, in some situations, the osteoblasts may be also labeled, such as when they display a phagocytotic activity in hypocalcemia.28
The teeth moved for 1 week did not show cells positive for TRAP staining on their root surfaces. This finding is in accordance with our previous scanning electron microscopy study in which no areas of resorbed cementum were observed after 1 week of torque application.23 However, the presence of TRAP-positive mononuclear cells at some distance from the smooth root surfaces at this early time point indicates that these cells may be precursors of clastic cells.
When the specimens moved for 2 weeks were examined, active clastic cells resorbed the root surface on the pressure sides because multinucleated TRAP-positive cells were detected at these areas. The TRAP-positive cells were confirmed by ultrastructural analysis, which revealed that these cells contained many vacuoles. It is interesting that the areas adjacent to the resorbing root surfaces consisted of hyalinized zones. This finding confirmed previous reports that clastic cells are located at the periphery of necrotic areas of the periodontal ligament.14 At longer application times, root resorption was severe and in some cases reached the underlying dentin. It is important to consider that, in contrast to the shallow concavities where only cementum was involved, complete repair usually did not occur when dentin was also resorbed.
Although all the clastic cells that resorb cementum, dentin, or enamel are generally referred to as odontoclasts,29 our results suggest that the particular composition of the tissue being resorbed may determine some structural characteristics of the clastic cells. For instance, all the active clastic cells observed in the present study were multinucleated. As mentioned above, mononuclear clastic cells are frequently observed near dentin during physiological tooth shedding,27 despite reports that multinucleated clastic cells were present during internal resorption of dentin.30 Thus, the composition of cementum appears to be somewhat different from that found in dentin31 and may be responsible for the apparent necessity for mononuclear precursors to fuse to form multinucleated clastic cells. This finding contradicts some previous reports that, by light microscopy, described cementum resorbing mononucleated cells.32–34
A surprising finding was the absence of a clear zone in the clastic cells. Conceptually, the clear zone is necessary for establishing an acidic microenvironment at the ruffled border region.8 Clastic cells lacking a clear zone have been detected on the surface of resorbed areas of enamel.7 Whereas sealing is mandatory for decreasing the pH at the central region, it is somewhat intriguing that enamel, which is almost exclusively mineral, may be resorbed without the necessity of a sealing zone. Despite these considerations, the possibility also exists that the absence of a well-defined clear zone in clastic cells could be because of the extreme thinness of the sections cut for transmission electron microscopy that limit the view of the cell to an 80-nm– thick section. This aspect is crucial in human studies in which a small sample size is necessary because of ethical reasons.
The applied force was continuous throughout the experiment as it was in our previous study.23 Although the biomechanics used elicited numerous areas of resorption, several repair areas were also observed on previously resorbed root surfaces. This confirms our previous findings14 indicating that repair of cementum also occurs during orthodontic continuous forces on human teeth. This aspect is important for the type of movement because, according to the classic concept, only intermittent forces could permit resting periods for periodontal tissue repair. However, this and our recent studies show that repair is not necessarily dependent on decreasing the applied force. This is also evident on the rat alveolar bone surface where osteoblastic activity is concomitant with the predominant resorption at the pressure area.35
CONCLUSIONS
-
Clastic cells and resorption lacunae were found at the pressure sides of roots.
-
The number of clastic cells and extent of resorption lacunae appear to increase with longer periods of applied force.
-
Tissue repair took place in some of the previously resorbed root surfaces even with the maintenance of the applied force.

Continuous torque moment for 1 week. Two mononuclear TRAP-positive cells (arrows) appear in the periodontal ligament (PL) far from the cementum (C), which shows no signs of resorption. Bar = 50 μm

Continuous torque moment for 2 weeks. A large multinuclear TRAP-positive clastic cell (CC) appears containing numerous granules at the center of the micrograph near the cementum (C) surface, while parts of others are seen at both sides. Bar = 10 μm

Continuous torque moment for 3 weeks. (a) Two multinucleated clastic cells (arrows) exhibit numerous TRAP-positive dark granules in a region of extravasation of red blood cells (r). Bar = 10 μm. (b) Another region of extravasation of red blood cells (r) adjacent to the cementum shows a multinucleated cell. C, cementum; arrows, vacuoles; m, mitochondria; N, nucleus. Bar = 4 μm

Continuous torque moment for 4 weeks. Two TRAP-positive clastic cells (arrows) appear inside a resorbing lacuna (between arrowheads) that has reached the root dentin (D) at the pressure side. C, cementum; asterisks, dentinocementum junction. Bar = 10 μm

Continuous torque moment for 4 weeks. (a) A multinucleated clastic cell exhibiting a well-developed ruffled border facing the resorbing cementum (C) contains TRAP-positive granules (arrows). r, red blood cells. Bar = 10 μm. (b) A multinucleated clastic cell (CC) with a clear ruffled border (arrowheads) is adjacent to the cementum. N, nucleus. Bar = 8 μm

Continuous torque moment for 4 weeks. (a) A multinucleated clastic cell containing TRAP-positive granules (arrows) and a well-developed ruffled border is apposed to the cementum (C). r, red blood cells. Bar = 10μm. (b) A clastic cell exhibiting a ruffled border (arrowheads) and vacuoles with a granular content (arrows) is discerned. Bar = 8 μm

Continuous torque moment for 3 weeks. (a) A TRAP-positive clastic cell (arrow) appears at the bottom of a deep resorbing lacuna at the pressure side. C, cementum; D, dentin; asterisks, dentinocementum junction; PL, periodontal ligament. Bar = 40 μm. (b) A cementoblast-like cell (arrowhead) is interposed between the clastic cell (arrow) and the root surface. Bar = 20 μm. (c) Some cementoblasts (CB) appear in contact with the cementum in a previously resorbed lacuna. Bar = 10 μm
Contributor Notes
Corresponding author: Professor Victor E. Arana-Chavez DDS, MSc, PhD, Laboratory of Mineralized Tissue Biology, Department of Cell and Developmental Biology, Institute of Biomedical Sciences, University of São Paulo, 05508-900 São Paulo, SP, Brazil (vearana@usp.br)